A clinical study was done to determine the safety and
effectiveness of REBLOZYL
The main goal of the study was to see if REBLOZYL could lower the number of transfusions needed by at least one-third during a set 12-week period compared to placebo.* People in both treatment groups were allowed to receive best supportive care. This included red blood cell (RBC) transfusions, iron-chelating agents, use of antibiotic, antiviral, and antifungal therapy or nutritional support, as needed.
*Set 12-week period was weeks 13–24.


Percentage of people who
REDUCED THE NEED FOR TRANSFUSIONS BY AT LEAST A THIRD
over 12 weeks of treatment
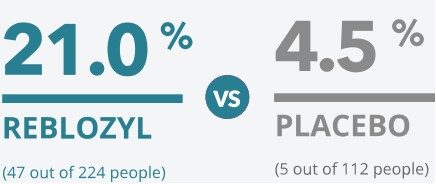
which means that
4x
greater percentage of patients receiving REBLOZYL reduced their transfusion burden vs placebo
REBLOZYL may mean fewer transfusions for you
While on REBLOZYL, you may lower the number of transfusions needed, which can look different from person to person. This is why it’s so important to talk about your treatment goals with your healthcare team. Together, you can make sure you’re tracking toward the same expectation.

Example patient: What this might look like

For example, if a patient needed 6 RBC units every 12 weeks before starting REBLOZYL…

…this patient may only need 4 (or less) RBC units during weeks 13 to 24 after starting REBLOZYL
REBLOZYL was studied in:

356 PEOPLE
with beta beta (β) thalassemia (BT)
who were transfusion dependent.

224 PEOPLE
received 1 REBLOZYL injection every 3 weeks
112 PEOPLE
received 1 placebo injection every week
Everyone in the study regularly received 6 to 20 units of packed RBCs and had not been transfusion-free for more than 35 days within 24 weeks before the study started
Patients could not be a part of the study if they were under 18 or had:
- Hemoglobin S/BT or alpha (α)-thalassemia
- Major organ damage (liver, heart, or lung disease, poor kidney function)
- Recent deep vein thrombosis, stroke, or use of erythropoiesis-stimulating agent, immunosuppressant, or hydroxyurea therapy
What are the possible side effects of REBLOZYL?
It’s important to know about the possible side effects of REBLOZYL before beginning treatment. Remember: people may react to medicines differently, and that’s expected.
The possible side effects of REBLOZYL are well-known. Be sure to talk with your healthcare team about what to expect when starting treatment with REBLOZYL.
What are the serious side effects of REBLOZYL?

Serious side effect
A serious side effect is a side effect that can sometimes become life-threatening and can lead to death. They may happen any time during treatment or even after your treatment has ended. You may experience more than one side effect at the same time.

Blood clots (thrombosis/thromboembolism)
Blood clots in the arteries, veins, brain, and lungs have happened in people with BT during treatment with REBLOZYL. The risk of blood clots may be higher in people who have had their spleen removed or who take hormone replacement therapy or birth control pills.
Call your healthcare provider or get medical help right away if you have any of these symptoms:
- Chest pain
- Trouble breathing or shortness of breath
- Pain in your leg, with or without swelling
- A cold or pale arm or leg
- Sudden numbness or weakness that is short-term or continues to happen over a long period of time, especially on one side of the body
- Severe headache or confusion
- Sudden trouble with seeing, speaking, balancing, walking, or dizziness

High blood pressure (hypertension)
REBLOZYL may cause an increase in your blood pressure. Your healthcare provider will check your blood pressure before you receive your REBLOZYL dose. Your healthcare provider may prescribe you medicine to treat high blood pressure or increase the dose of medicine you already take to treat high blood pressure, if you develop high blood pressure during treatment with REBLOZYL.

Extramedullary Hematopoietic (EMH) Masses
EMH masses have happened in people with BT during treatment with REBLOZYL. You may have a higher risk for developing EMH masses if you have a history of EMH masses, have had your spleen removed, have or have had enlarged spleen or liver, or have low hemoglobin levels. Your healthcare provider will monitor you before you start and during treatment with REBLOZYL. Call your healthcare provider or get medical help right away if you get any of these symptoms:
- Severe pain in the back
- Numbness or weakness or loss of voluntary movement in feet, legs, hands, or arms
- Loss of bowel and bladder control
What are the most common side effects of REBLOZYL?
Most common side effects
The most common side effects are the side effects that were the most frequently reported by patients in the study. You may experience more than one side effect at the same time.
The most common side effects of REBLOZYL include:
- tiredness
- headache
- back, joint, muscle, or bone pain
- joint pain
- dizziness
- nausea
- diarrhea
- cough
- stomach (abdominal) pain
- trouble breathing
- swelling of your hands, legs, or feet
- high blood pressure
- allergic reactions
These are not all of the possible side effects. For more information about side effects, please see the REBLOZYL Patient Information.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.


